- This topic is empty.
-
AuthorPosts
-
26/11/2025 at 18:08 #6626
2-(Tributylstannyl)furan (CAS No.: 118486-94-5) is a highly valuable organotin compound widely used in modern organic synthesis, pharmaceutical development, and materials science. Known for its strong nucleophilic activity and chemical stability under neutral conditions, this compound has become an essential intermediate in the synthesis of bioactive organotin derivatives.
With a molecular formula of C₁₆H₃₀OSn and a molecular weight of 357.12, 2-(Tributylstannyl)furan appears as a colorless liquid under normal conditions. It exhibits a density of 1.134 g/mL at 25 °C, a boiling point of 90 °C at 0.1 mmHg, a refractive index of n²⁰/D 1.494, and a flash point exceeding 230 °F. For optimal stability, it should be stored in an inert atmosphere at room temperature, as prolonged exposure to air may lead to gradual oxidation. In this blog post, SACH, as a high purity organotin compound manufacturing factory, will share the versatile applications of 2-(tributylstannyl)furan in chemistry.
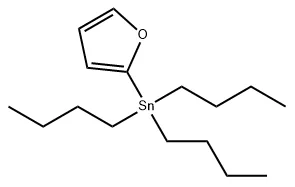
Physicochemical Characteristics of 2-(Tributylstannyl)furan
At room temperature and pressure, 2-(Tributylstannyl)furan (CAS No.: 118486-94-5) exists as a colorless and slightly viscous liquid. It is insoluble in water, but soluble in many organic solvents such as benzene, dichloromethane, and petroleum ether.
Chemically, the compound exhibits high nucleophilicity, allowing it to readily participate in various organic transformations. It reacts efficiently with halogens, anhydrides, acid chlorides, and other electrophilic compounds to produce a range of organotin derivatives. Furthermore, it can react with other metal-organic compounds, forming new organotin structures with tailored reactivity.
This balance of stability and reactivity makes it an ideal choice for use as both a synthetic intermediate and a catalyst component in advanced organic chemistry.
Synthesis of 2-(Tributylstannyl)furan
Method 1: Direct Reaction of Tributyltin Chloride and Furan
A classical synthetic route involves refluxing tributyltin chloride and furan for approximately ten hours. After the reaction, the mixture is filtered, and the filtrate is concentrated under reduced pressure. The addition of petroleum ether induces precipitation, followed by recrystallization with dichloromethane–petroleum ether. The resulting product—after repeated washing and purification—is a colorless viscous liquid, identified as 2-(Tributylstannyl)furan.
Method 2: Synthesis in Dehydrated Benzene Medium
In another route, tributyltin chloride and furan are introduced into anhydrous benzene and refluxed for five hours under stirring. After cooling and filtration, the solvent is evaporated under reduced pressure to dryness. The crude product is purified through acetone–petroleum ether recrystallization, yielding a pure, colorless liquid suitable for further applications.
Both methods offer high yields and purity when conducted under controlled inert conditions, ensuring minimal oxidation and maintaining the compound’s structural integrity.
Applications of 2-(Tributylstannyl)furan in Organic Synthesis
1. Organic Synthesis Intermediate
2-(Tributylstannyl)furan is a versatile synthetic building block in organotin chemistry. It serves as a key intermediate for producing a variety of tributyltin carboxylates, dithiophosphonates, and related derivatives. Many of these compounds exhibit bactericidal, herbicidal, and insecticidal properties.
Due to its controllable reactivity and high purity (98% standard), this intermediate is widely used in laboratory and industrial synthesis processes that aim to develop biologically active organotin molecules with enhanced efficiency and environmental compatibility.
2. Role in Agrochemical Development
In the agrochemical field, 2-(Tributylstannyl)furan is utilized to synthesize low-toxicity, biodegradable pesticide intermediates. These include tributyltin-based fungicides and insecticides with broad-spectrum activity against a range of crop pathogens and pests.
Such compounds have become essential in modern sustainable agriculture, offering an effective solution for pest management while reducing chemical residues and environmental pollution.
3. Pharmaceutical Intermediate for Bioactive Organotin Compounds
2-(Tributylstannyl)furan plays a significant role in pharmaceutical synthesis, particularly in the creation of organotin compounds with anti-tumor, antiviral, and antibacterial activities. Its strong nucleophilic properties enable it to form carbon–tin bonds that serve as reactive centers for further chemical modification.
By integrating 2-(Tributylstannyl)furan into multi-step synthetic routes, researchers can efficiently design new drug candidates or bioactive intermediates with precise molecular configurations.
4. Catalyst in Advanced Organic Reactions
As an organotin derivative, 2-(Tributylstannyl)furan also exhibits catalytic properties in certain organic transformations. It is particularly useful in coupling and cyclization reactions, where it can enhance reaction rates and improve selectivity.
For example, it is often used in Stille coupling reactions, facilitating carbon–carbon bond formation between organotin compounds and organic halides. This catalytic capability makes it a vital component in synthetic chemistry research and industrial production of fine chemicals.
5. Applications in Materials Science
Beyond chemical synthesis, 2-(Tributylstannyl)furan finds applications in materials science. It can serve as a precursor for creating organotin-based conductive or optical materials, which are of growing interest in electronics and photonics.
Its ability to form tin–carbon linkages with precise structural control enables the production of materials with unique electronic, thermal, or optical properties, offering potential for use in semiconductors, sensors, and thin-film technologies.
Storage and Handling Guidelines
For optimal stability, 2-(Tributylstannyl)furan should be stored under an inert atmosphere such as nitrogen or argon. Containers should be tightly sealed and kept at room temperature in a dry, well-ventilated environment. Avoid exposure to air, moisture, and direct sunlight, as these can cause oxidation or degradation over time.
The compound is supplied in 1 kg drums, ensuring safe handling and convenience for laboratory and industrial use. Always observe standard safety protocols when handling organotin compounds, including the use of gloves, goggles, and fume hoods.
Conclusion
2-(Tributylstannyl)furan (CAS No.: 118486-94-5) stands as a cornerstone compound in modern organotin chemistry, offering an ideal balance between stability and reactivity. Its broad applications—from organic synthesis and catalysis to agrochemicals, pharmaceuticals, and materials science—demonstrate its strategic importance in both research and industry.
As the demand for environmentally friendly and high-performance chemicals continues to rise, 2-(Tributylstannyl)furan is expected to play an increasingly crucial role in the innovation of new-generation organotin materials and bioactive compounds.
-
AuthorPosts
- You must be logged in to reply to this topic.